Intracellular Delivery of mRNA for Cell-Selective CRISPR/Cas9 Genome Editing using Lipid Nanoparticles
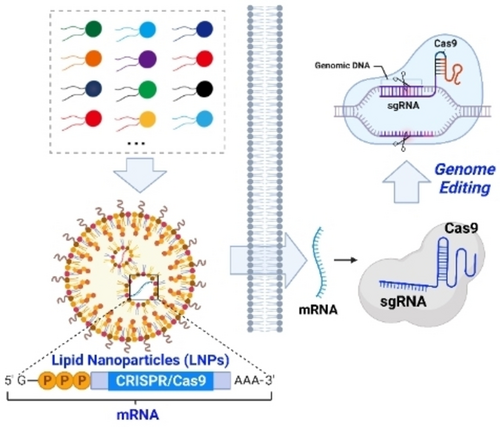
Graphical Abstract
The optimal delivery of mRNA by using lipid nanoparticles (LNPs) can be achieved through a rational design process and high-throughput screening of a lipid library. The spatiotemporally controlled delivery of mRNA-encoding CRISPR/Cas9 can provide an effective and convenient approach for cell-selective genome editing and targeted gene therapy.
Abstract
Messenger RNA (mRNA) is being used as part of an emerging class of biotherapeutics with great promise for preventing and treating a wide range of diseases, as well as encoding programmable nucleases for genome editing. However, mRNA's low stability and immunogenicity, as well as the impermeability of the cell membrane to mRNA greatly limit mRNA's potential for therapeutic use. Lipid nanoparticles (LNPs) are currently one of the most extensively studied nanocarriers for mRNA delivery and have recently been clinically approved for developing mRNA-based vaccines to prevent COVID-19. In this review, we summarize the latest advances in designing ionizable lipids and formulating LNPs for intracellular and tissue-targeted mRNA delivery. Furthermore, we discuss the progress of intracellular mRNA delivery for spatiotemporally controlled CRISPR/Cas9 genome editing by using LNPs. Finally, we provide a perspective on the future of LNP-based mRNA delivery for CRISPR/Cas9 genome editing and the treatment of genetic disorders.
1 Introduction
Messenger RNA (mRNA) serves as a transient carrier of genetic information, transcribed from DNA, that can be translated into protein to modulate cellular function and fate.1 Since its discovery in 1961,2 the biomedical and therapeutic potential of mRNA has been extensively explored, yet challenges remain due to the low stability, immunogenicity, and impermeability of mRNA through the cell membrane.3 Malone et al. were the first to demonstrate successful expression of targeted protein in cells by delivering exogenous mRNA using the cationic lipid DOTMA,4 this discovery laid the foundation for the use of exogenous RNA as biotherapeutics. However, at the time, the therapeutic potential of mRNA was greatly limited by the difficulty of producing mRNA on a large scale. In 1984, Melton et al. reported the preparation of biologically active mRNA using an in vitro transcription strategy (IVT),5 which greatly enhances the therapeutic potential of mRNA. There are several significant advantages to using mRNA as therapeutics compared to conventional DNA-based gene therapy. Firstly, mRNA is translated immediately upon entering the cytoplasm, bypassing the need to enter the nucleus, resulting in fast protein translation. Secondly, mRNA-based gene therapy is safer due to the low risk of integration into the host cell genome, as compared to conventional gene therapies that deliver DNA using viral nanoparticles.6 Therefore, mRNA holds great promise for developing biotherapeutics for treating a large variety of genetic disorders, including protein replacement therapy,7 mRNA vaccines,8, 9 immunotherapies,10 and gene editing.11
mRNA is a large, negatively charged biomacromolecule that faces significant challenges in attempting to penetrate cell membranes spontaneously. In addition, mRNA is prone to rapid degradation in various biological settings, such as blood and other body fluids.12 To enable clinical translation of mRNA, appropriate delivery vehicles are necessary to protect mRNA from degradation and promote its delivery to target cells and tissues. Due to the challenges of mRNA delivery, viral vectors like adeno-associated vectors (AAV) have shown high efficiency in delivering mRNA.13 However, the potential immunogenicity of viral nanoparticles may cause safety concerns for the clinical translation of mRNA, particularly in pediatric indications.14 Therefore, nonviral delivery methods, such as LNPs,15 gold nanoparticles,16 polymeric nanoparticles17, 18 and mesoporous silica nanoparticles19, 20 have emerged as promising alternatives for mRNA delivery due to their safety and ease of synthesis. Among these mRNA delivery systems, LNPs have received significant attention due to their high efficiency and safety profile. Recently, LNPs have been clinically approved for the development of mRNA-based vaccines for the prevention of COVID-19, demonstrating their potential as a versatile platform for mRNA delivery.21
In this review, we provide a summary of the latest progress in designing ionizable lipids and their formulations for intracellular mRNA delivery, with a focus on delivering mRNA in a cell- and tissue-specific manner. We will also discuss the use of LNPs-mediated mRNA delivery for spatiotemporally controlled CRISPR/Cas9 genome editing, and provide a perspective on future studies of mRNA delivery for CRISPR/Cas9 genome editing and genetic disorder treatment.
2 Chemical Modification of mRNA for Intracellular Delivery
The structure of mRNA prepared through IVT typically consists of five functional domains, which include the 5′-cap, untranslated regions (UTRs) located at the 5′- and 3’-ends, the open reading frame (ORF) that encodes the protein of interest, and a poly(A) tail (Figure 1). Recent research has demonstrated that optimizing the structural domains of mRNA can significantly enhance both its intracellular stability and translation efficiency.22 Eukaryotic mRNA typically contains a 7-methylguanosine (m7G) cap, which is linked to the mRNA by a 5′–5′-triphosphate bridge to form a m7GpppN structure. The capping of mRNA has been shown to protect it from degradation and enhance its binding with eukaryotic translation initiation factor4E (eIF4E), which is essential for mRNA translation.23 In the past few decades, an anti-reverse cap analogue containing an O-methyl or deoxy group at C2′ to prevent capping in the reverse orientation has been widely utilized for mRNA capping. This modification has been found to enhance mRNA stability and translation efficiency.24 The use of ARCA cap analogues containing a phosphorothioate has been recently designed to enhance the stability of mRNA and prolong its half-life, in order to resist mRNA degradation.25, 26 Due to the limited translation efficiency and relatively high immunogenicity of ARCA capping, further improvements can be achieved by converting Cap-0 into Cap-1 through O-methylation of the ribose of the cap-proximal nucleotide,27 which was used in the mRNA-based vaccine against SARS-CoV-2.28
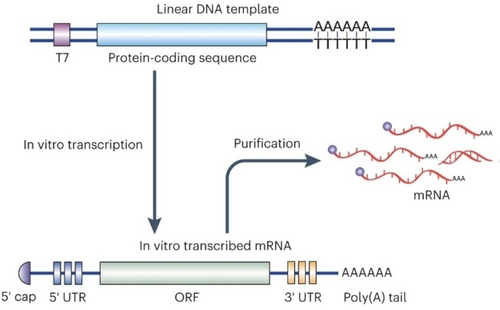
Schematic illustration of in vitro transcription (IVT) of mRNA. mRNA is synthesized in vitro by using a linear DNA template and RNA polymerase (T7). The IVT mRNA is composed of five domains: 5′-cap, 5′- and 3’-UTR, an ORF encoding the protein of interest, and a poly(A) tail. Reproduced with permission from ref. 22. Copyright: 2022, Nature Publishing Group.
The sequence of the 5′- and 3′-UTRs can further regulate mRNA stability and translation efficiency. For example, the unique 3′ UTR sequence of human β-globin mRNA confers high stability, making it a useful sequence for optimizing mRNA stability and translation efficiency.29 The mRNA stabilizing effect of 3′-UTR sequence of Human β-globin can be further enhanced by using two human β-globin 3-UTR sequence arranged in a head-to-tail orientation.30 However, UTR sequence optimization is typically necessary to improve mRNA stability and expression efficiency in a customized manner. For example, the UTR sequence of the human CYBA gene has been shown to increase the expression of human BMP2 mRNA in NIH3T3 and A549 cells.31 Zhang et al. investigated the crucial role of the 5′UTR sequence on green fluorescent protein (GFP) expression in HEK293T cells. The study demonstrated that the highest level of fluorescent protein expression was achieved using the 5′-UTR sequence of the chEF1 gene.32 The UTR sequence of mRNA can now be optimized using a high-throughput screening method33 or deep learning strategy.34 For the design of mRNA encoding nucleases for genome editing, it might be necessary to regulate the stability of the mRNA to limit the duration of the nuclease inside cells. This effect can be achieved by incorporating AU-rich elements into the 3′-UTR to allow for rapid mRNA degradation and a short duration of protein expression.35
The addition of a poly(A) tail to the 3′-end of mRNA, typically containing 100–150 nucleotides, can improve its stability and translational efficiency. Poly(A) tails form complexes with poly(A) binding protein, which aids in the initiation of mRNA translation.36 Messenger RNA containing a poly(A) tail longer than 100 nt typically exhibits high translation efficiency. However, DNA templates containing such a long poly(A) segment are unstable for in vitro transcription. Therefore, the addition of a short UGC linker to the poly(A) tail can improve its stability.37 The mRNA-based vaccine against SARS-CoV-2 developed by Pfizer-BioNTech, BNT162b2 uses mRNA containing a 10 bp UGC linker in the poly (A) tail.
The nucleosides in natural eukaryotic mRNAs are chemically modified, including pseudouridine (ψ), 5-methylcytidine, N6-methyladenine, 5-methyluracil, and 2-thiouracil. These modifications can reduce the recognition of mRNA by human TLRs, leading to a decrease in the production of type I interferons (IFNs) and proinflammatory cytokines, such as tumor necrosis factor-α (TNF-α), IL-6, and IL-12.38 In addition, N1-methylpseudouridine modification of mRNA shows a lower cytotoxicity and immune-stimulative efficiency,39 it therefore has been used in the COVID-19 vaccine to avoid unexpected immune responses developed by Moderna and Pfizer-BioNTech.
3 Intracellular Delivery of mRNA by Using LNPs
The basic structure of LNPs comprises ionizable lipids, cholesterol, helper phospholipids, and PEGylated lipids. These components form a self-assembled structure that allows mRNA to be encapsulated within the internal cavity, thereby improving the stability of mRNA during intracellular delivery. LNPs exhibit protonation under acidic intracellular conditions while remaining neutral under physiological pH, thereby reducing their physiological toxicity and extending their half-life for in vivo mRNA delivery.40 The composition and proportion of LNPs, nanoparticle size, and surface charge are all critical factors that have been shown to affect mRNA delivery and in vivo targeting efficiency in previous studies.41-44
The chemical structure of ionizable lipids plays an essential role in encapsulating mRNA and determining mRNA delivery efficiency,45, 46 therefore there is a great need to discover new types of lipids for mRNA delivery. In recent years, a strategy involving the design of a combinatorial library of lipids, followed by high-throughput screening, has been used to discover potent ionizable lipids for mRNA delivery and to study the structure–activity relationship. Wang and Xu et al. designed a combinatorial library of ionizable lipids bearing unsaturated hydrophobic tails through the Michael addition reaction of aliphatic amines and oleyl acrylamide. Compared with saturated lipids, they found that unsaturated lipids show increased membrane fluidity to enhance mRNA delivery efficiency. While obtaining the optimal lipid 16-I, they reported that the chemical structure of aliphatic amines used for lipid synthesis greatly affected mRNA delivery efficiency (Figure 2).47 Seigwart et al. investigated the structure–activity relationship of unsaturated LNPs for mRNA delivery and concluded that introducing unsaturated bonds to the lipid tail might not be a universal strategy to enhance mRNA delivery efficiency. Specifically, they found that the location of unsaturated bonds in the lipid tail and the cis−trans conformation of unsaturated bonds are more crucial in determining mRNA delivery efficiency. They further identified lipid 4 A3-Cits containing citronellol as the optimal LNP for mRNA delivery (Figure 2).48 Anderson et al. designed an ionizable lipid, A6, containing alkyne bonds for targeted delivery of mRNA to hepatocytes. Their study suggested that the addition of triple bonds may provide higher membrane fusion efficiency than double bonds. They found that human erythropoietin (hEPO) mRNA encapsulated by A6 can almost completely cure renal anemia in mice, demonstrating that these LNP formulations synergistically facilitate robust mRNA delivery with improved tolerability after single and repeated administrations (Figure 2).49 Wang and Xu et al. further reported that by introducing disulfide bonds into the hydrophobic tail of ionizable lipids, the as-designed bio-reducible LNPs can be degraded by intracellular glutathione to promote endosomal escape of LNPs and the release of mRNA inside cells.50 They have further screened and optimized LNPs for lymph node-targeted mRNA delivery using lipid 113-O12B, which can deliver mRNA to 30 % of antigen-presenting cells (Figure 2).51 We have recently designed a novel type of reactive oxygen species (ROS)-degradable LNPs containing a thioketal (TK) moiety that enables enhanced delivery of mRNA to tumor cells. It has been previously reported that the intracellular ROS level in cancer cells is about 5000 times higher than that of normal cells,52 optimal ROS-degradable LNPs, BAmP-TK-12, demonstrated one-fold more potent mRNA delivery and target protein expression in cancerous cells compared to normal cells, as shown in Figure 2. Furthermore, BAmP-TK-12 was able to effectively deliver bacterial effector protein DUF-encoding mRNA to deplete mutant RAS in a wide range of cancer cells. This outperformed the clinically approved RAS-targeting small molecule, AMG510, for inhibiting tumor cell growth both in vitro and in vivo (Figure 3A).15
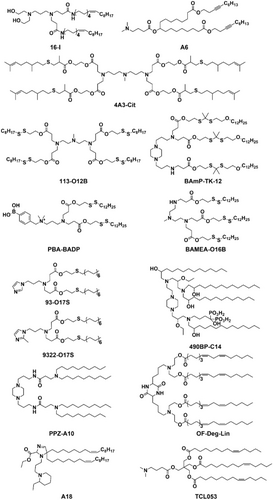
Chemical structure of ionizable lipids that have been recently designed for mRNA delivery.
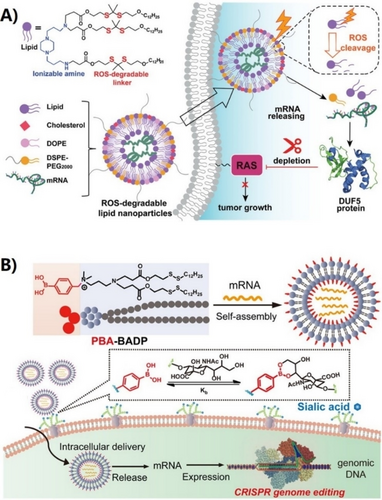
A) Schematic illustration of ROS-responsive lipid nanoparticles, BAmP-TK-12 for mRNA delivery for mutant RAS depletion and potential cancer therapy. Reproduced with permission from ref. 15. Copyright: 2022, Wiley. B) Schematic illustration of assembling PBA-BADP/mRNA nanoparticles for cancer-cell-targeted mRNA delivery and CRISPR/Cas9 genome editing. Reproduced with permission from ref. 54. Copyright: 2019, American Chemical Society.
Targeted delivery of mRNA to specific organs or cells can improve the therapeutic efficacy and reduce side effects of mRNA, especially for genome editing applications. Targeted mRNA delivery using LNPs can be achieved by designing the surface of LNPs with a large variety of ligands, including small molecules, aptamers, monoclonal antibodies, and peptides to interface specific receptors on cells.43 For example, Rurik et al. reported that the modification of LNPs surface with antibodies to target T cells can deliver mRNA to reprogram T cells to specifically attack overactive cardiac fibroblasts, providing a new approach for treating heart failure.53 The conjugation of targeting ligands with ionizable lipids provides a convenient and efficient approach for delivering mRNA in a selective manner, particularly for non-liver targeted delivery. For instance, due to the overexpression of sialic acid (SA)-modified protein on the surface of cancer cells, LNPs composed of phenylboronic acid (PBA)-modified ionizable lipid showed enhanced cellular uptake efficiency via PBA/SA interactions, enabling cancer cell-targeted mRNA delivery using PBA-BADP LNPs (Figures 2 and 3B).54 Similarly, Mitchell et al. recently designed a library of LNPs containing alendronate, in which the bisphosphonates can chelate with calcium ions for bone-targeted mRNA delivery. They demonstrated that the delivery of bone morphogenetic protein-2 (BMP-2)-encoding mRNA using 490BP-C14 LNPs resulted in BMP-2 protein secretion on the bone surface and in the bone marrow (Figure 2).55
Xu et al. reported that LNPs containing imidazole could deliver mRNA specifically to primary T lymphocytes. They identified two types of LNPs, composed of 93-O17S and 9322-O17S, which could target the spleen for mRNA delivery, allowing the reprogramming of CD4+ and CD8+ T lymphocytes. This targeted mRNA delivery could have important implications for immunotherapy, as it enables the engineering of immune cells to fight diseases like cancer or viral infections.56 Dahlman et al. reported that LNPs containing a piperazine-derived lipid, PPZ-A10, could preferentially deliver mRNA to immune cells in the liver and spleen, even at a low dose of 0.3 mg/kg (Figure 2). However, it is important to balance the increased mRNA encapsulation efficiency and the size of the nanoparticles, as the hydrophobic chain length of the lipids increases.57 Peer et al. found that lipids containing piperazine head group can deliver mRNA and accumulate efficiently in spleen, while lipids composed of tertiary amine-based head group mostly deliver mRNA to liver.58 Anderson et al. found that the incorporation of nitrogen-containing heterocyclic rings in the head of LNPs enhanced mRNA delivery efficiency and increased IFN-γ secretion up to 75-fold compared to linear ones. These heterocyclic rings, such as piperidine and azepane, were found to activate the cGAS-STING-type I IFN signaling pathway. In particular, dihydroimidazole exhibited the highest binding capacity with STING C-terminal domain binding pocket. The use of A18 LNPs containing dihydroimidazole to deliver mRNA vaccines resulted in potent anti-tumor effects, with approximately 50 % of B16-OVA melanoma animal models treated with A18 LNPs surviving more than 40 days(Figure 2).59 Recently, they found that OF-Deg-Lin LNPs can deliver mRNA specifically to spleen B lymphocytes,60 unlike most ester-based LNPs which mainly deliver mRNA to the liver, due to the organ-dependent degradation of OF-Deg-Lin ester linkers (Figure 2).61 Dong et al. synthesized vitamin-derived lipid nanoparticles (VLNPs) by combining vitamins or their derivatives with amino lipids. The VLNPs enable specific delivery and accumulation of antimicrobial peptide and cathepsin B-encoding mRNA in macrophages.62
The outer surface of nanoparticles undergoes a rapid process of adsorption of a thin layer of serum proteins, which is commonly referred to as the “protein corona”, subsequent to intravenous administration. This protein corona causes significant changes in the surface properties of nanoparticles and significantly impacts their interactions with organs and cells, thereby influencing the in vivo targeting of mRNA delivery.63 LNPs are known to adsorb apolipoprotein E after intravenous administration, leading to liver-targeted delivery via binding to the low-density lipoprotein receptor expressed on hepatocytes. However, recent findings by Xu et al. suggest that LNPs with amide bonds as the linkage between head amine and hydrophobic tail can adsorb different serum proteins such as albumin, fibrinogen β chain, and γ chain, allowing for lung-targeted mRNA delivery. Interestingly, the protein corona composition of liver and lung-targeting LNPs showed significant differences in molecular weight and isoelectric point distribution, which were independent of the surface charge of LNPs. These results suggest that the introduction of amide bond as the linker of head group and hydrophobic tail of lipids can regulate the composition of the LNPs protein corona, enabling lung-targeted mRNA delivery using LNPs.64 In a study by Dahlman et al., it was demonstrated that changing the hydrophobic tail length of 2,5-piperazinedione-derived lipids enables RNA delivery in an ApoE- and LDLR-independent manner, suggesting the determinative role of the chemical structure of lipids on the trafficking pathway of LNPs.65
Siegwart et al. recently presented a predictive design for LNPs, known as SORT, that enables tissue-specific mRNA delivery and genome editing by controlling the protein corona on the LNP surface. This approach involves the addition of a fifth lipid, called the SORT molecule, to the traditional four-component LNPs, which regulates the protein corona on the LNP surface and allows for targeted delivery of mRNA and genome editing in the liver, spleen, and lung. SORT contains additional charged lipids, which regulate the total/apparent pKa of LNPs and affect the isoelectric points of the major proteins bound to LNPs. While liver SORT was found to most avidly bind ApoE, spleen and lung SORT LNPs enriched β2-glycoprotein I and vitronectin at their surfaces, respectively, indicating an ApoE-independent mechanism for extrahepatic mRNA delivery. Therefore, the addition of quaternary amine-containing cationic SORT molecules provides a lung-targeting effect, while phosphoric acid-containing negatively charged SORT lipids enable LNPs to target the spleen, and ionizable SORT lipids can further enhance the enrichment of mRNA nanoparticles in the liver. Importantly, the SORT strategy demonstrates high generality in regulating the targeting effect of various four-component LNPs for delivering mRNA, Cas9 mRNA/sgRNA, and Cas9 ribonucleoprotein complexes for genome editing (Figure 4).66-68
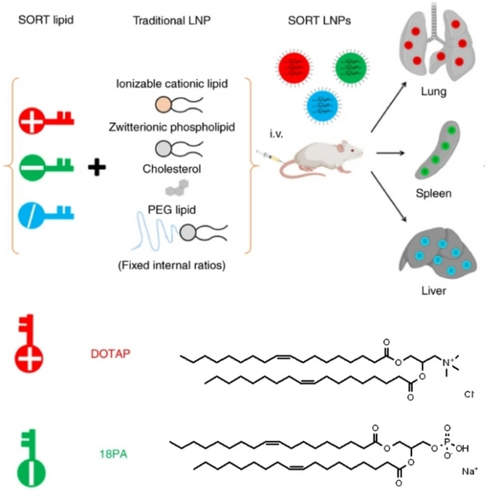
Schematic illustration of the SORT strategy, and chemical structure of permanently cationic lipid DOTAP, anionic lipid 18PA as SORT molecule for tissue-specific mRNA delivery and CRISPR/Cas9 genome editing. Reproduced with permission from ref. 66. Copyright: 2020, Nature Publishing Group.
4 Intracellular Delivery of CRISPR mRNA for Cell-Selective Genome Editing
Since its discovery in 2012, the CRISPR/Cas9 genome-editing technology has rapidly become a powerful and convenient tool for chemical biology and biomedical research. Its ability to modify and correct genomic DNA has made it an essential component of the modern molecular toolbox.69-71 CRISPR/Cas9 genome editing relies on the use of a single-guide RNA (sgRNA) to recognize target DNA, directing Cas9 nuclease to introduce site-specific double-strand breaks (DSB) at target gene loci.72 However, effective genome editing in mammalian systems using CRISPR/Cas9 relies on the efficient intracellular delivery of Cas9 and sgRNA. In the past several years, there has been great success in designing nonviral nanoparticles to deliver CRISPR genome editing tools in the form of DNA plasmids, mRNA, or ribonucleoprotein.73, 74
Genomic DNA DSBs induced by CRISPR/Cas9 can lead to a large number of random insertions and deletions at target sites, as well as p53-dependent cytotoxicity,75 large DNA deletions, and complex rearrangements in the genome.76, 77 The emergence of base editing has made it possible to precisely convert one target DNA base into another without requiring double-stranded DNA backbone cleavage.78, 79 By fusing the Cas9 nickase with a cytosine/adenine deaminase enzyme, the cytidine/adenine base editor is capable of efficiently performing four base transitions. In addition, Liu et al. developed the prime editor (PE), which can achieve whole 12-base conversions, as well as insertion and deletion mutations.80 Prime editing is a revolutionary approach to genome editing, which employs a fusion protein of dCas9 endonuclease and engineered reverse transcriptase to directly introduce new genetic information, guided by a specific RNA molecule, into a targeted site in DNA. By avoiding the need for DSB formation, Prime editing significantly expands the range of genetic modifications that can be performed with high precision. In theory, Prime editing has the potential to correct up to 89 % of known genetic variations linked to human disease, representing a major advance in the field of gene therapy.
The off-target effects associated with CRISPR/Cas9 technology present a risky challenge for gene-editing tools. These effects may be caused by nonspecific binding between sgRNA and genomic DNA, and the prolonged presence of Cas9 nuclease delivered in the format of DNA. As a result, the CRISPR/Cas9 genome editing machinery is only needed temporarily until the desired genome modification occurs. Thus, it is highly desirable to reduce the risk of off-target editing by regulating the activity of CRISPR RNA delivery.81-83 In contrast, delivering CRISPR RNA by using LNPs might provide cell-selective targeting and enable genome editing in specific cell types, thus reducing the risk of off-target effects.84 To increase the specificity of CRISPR RNA delivery and reduce off-target effects, recent studies have focused on the design of biodegradable LNPs with cell-selective targeting. For example, the introduction of disulfide bonds into the hydrophobic tail of ionizable lipids has been shown to promote endosomal escape and intracellular release of Cas9 mRNA, while also allowing for degradation under the action of intracellular glutathione. These bio-reducible LNPs have shown promise for CRISPR RNA delivery with improved specificity and reduced off-target effects.85 This strategy was further expanded to achieve co-delivery of Cas9 mRNA and sgRNA for gene editing, both in vitro and in vivo, targeting PCSK9. This approach shows great potential for the treatment of cardiovascular diseases (Figure 5).86
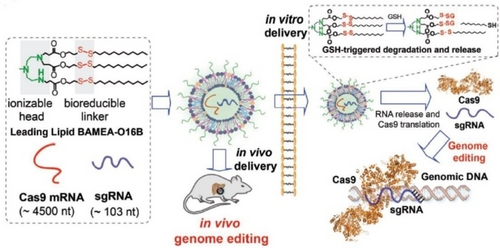
Schematic illustration of the bio-reducible lipid nanoparticle BAMEA-O16B for mRNA delivery and CRISPR/Cas9 genome editing delivery in vitro and in vivo. Reprinted with permission from ref. 86. Copyright: 2019, Wiley.
The conditional expression of Cas9 or activation of Cas9 variants using chemical agents87, 88 or external triggers89 has been shown to be effective in reducing off-target effect. However, the efficacy of these approaches for disease cell-selective and in vivo genome editing is still limited. Notably, the delivery of CRISPR mRNA using tissue-targeted LNPs has shown potential for improving the selectivity of genome editing. Siegwart et al. demonstrated that SORT LNPs can be used to co-deliver Cas9 mRNA and sgRNA in a tissue-specific manner, enabling specific gene editing in the liver, lung, and spleen.66 Hotta et al. reported that the TCL053 LNP has the ability to target mouse skeletal muscle for delivering the CRISPR system to treat Duchenne muscular dystrophy (DMD), and its therapeutic effect can be sustained for several months or longer, as long as the edited cells remain viable (Figure 2).90 Recently, we developed a novel strategy for delivering Cas9 mRNA specifically to tumor cells using phenylboronic acid-conjugated LNPs (PBA-BADP). This approach has been used to knock out the HPV18E6 gene in tumor cells, with the aim of developing a new cancer therapy.54 Peer et al. demonstrated the potential of CRISPR-LNPs (cLNPs) for the delivery of CRISPR mRNA to treat cancer. They found that L8-cLNP, derived from DLin-MC3-DMA, can deliver CRISPR RNA with high specificity to tumors, resulting in effective editing of PLK1. This led to G2-M cell cycle arrest and inhibition of tumor cell growth. A single intravenous administration of PLK1-cLNPs resulted in about 70 % gene editing and extended the median survival of mice by 50 %.91
More recently, we reported that intracellular delivery of activatable CRISPR systems using biodegradable LNPs enables tumor-targeted genome editing both in vitro and in vivo. We first developed a conditional and cell-selective genome editing system, enzyme-inducible CRISPR/Cas9 (eiCRISPR), controlled by disease-associated enzymes. eiCRISPR contains Cas9 mRNA, a self-blocked sgRNA (bsgRNA) that shows prohibited genomic RNA recognition ability, and a chemically caged DNAzyme. The DNAzyme was designed to cleave bsgRNA and activate CRISPR in a sequence-specific manner. However, chemical caging or modification of DNAzyme prohibits its ability to cleave bsgRNA due to the blocking of the essential active site of DNAzyme. When eiCRISPR was delivered into tumor cells using ROS-degradable LNPs, BAmP-TK-12, disease-related endogenous chemical signals or enzymes can selectively activate DNA enzymes, cleaving bsgRNA to restore the genome editing ability of eiCRISPR. The rational design of chemically caged DNAzyme allows the activation of eiCRISPR in an orthogonal manner by enzymes overexpressed in cancer cells, thus enabling cell-selective genome editing. As a proof-of-concept study, NAD(P)H: quinone oxidoreductase (NQO1) overexpressed in tumor cells can activate 17E-QP DNAzyme, enabling cancer cell-selective genome editing.92 In vivo delivery of eiCRISPR using BAmP-TK-12 LNPs enabled delivery and accumulation of eiCRISPR at the tumor site for in vivo activation and HPV18 E6 editing for cancer therapy. The injection of eiCRISPR nanoparticles containing 17E-QP reduced tumor volume to 30 % of the control group, and the indel efficiency was determined to be around 20.1 %. The combination of LNPs-mediated CRISRP RNA delivery and orthogonal activation of eiCRISPR in response to the disease cell environment allows cell-selective gene editing. This strategy reduces the negative effects of genome editing technology and improves the therapeutic potential of genome editing technology (Figure 6).11
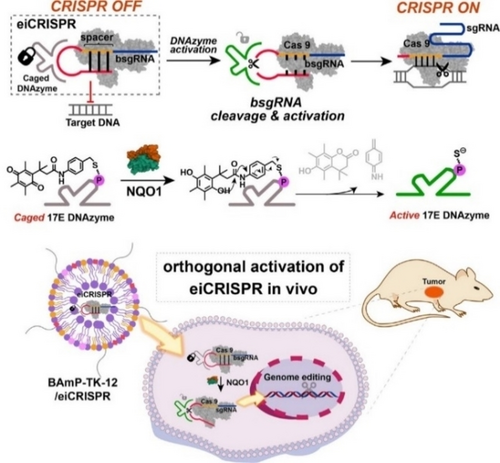
Schematic illustration of eiCRISPR and its intracellular delivery using LNPs for cell-selective genome editing. Chemical caging of DNAzyme enables its enzymatic activation by NQO1 overexpressed in tumor cells to activate bsgRNA and CRIPSR for cell-selective genome editing. Reproduced with permission from ref. 11. Copyright: 2022, American Chemical Society.
5 Conclusions and Outlook
The recent advances in intracellular delivery of mRNA by using LNPs indicate the great potential of harnessing mRNA to prevent infectious diseases and treat genetic diseases. So far, LNPs are the most studied and mature mRNA delivery systems, as evidenced by their clinical approval for developing mRNA-based vaccines for the prevention of COVID-19. The inherent accumulation of LNPs in the liver makes them a promising and attractive delivery platform for treating liver disease. In 2020, the first clinical trial of CRISPR/Cas9 technology using LNP-based mRNA delivery was initiated by Intellia Therapeutics. NTLA-2001, administered by intravenous administration, is a CRISPR/Cas9 gene therapy targeting the TTR gene in hepatocytes for the treatment of hereditary transthyroxin amyloidosis with polyneuropathy. A single administration of NTLA-2001 can reduce the level of key pathogenic proteins in plasma by about 90 %, with the therapeutic effect being maintained for a long time, thus demonstrating its potential to cure previously incurable genetic disorders.93 Intellia Therapeutics announced in November 2021 the launch of its second clinical trial, which aims to prevent angioedema attacks in patients with hereditary angioedema. The trial involved administering NTLA-2002 to patients, and after a single dose, the therapeutic effect lasted for 5–10 months. The trial did not observe any dose-limiting toxicity, thus indicating the potential safety and effectiveness of this approach.94
Although CRISPR/Cas9 genome editing technology offers a powerful approach for modifying the genetic material of mammalian cells, concerns about its use remain, particularly with regard to possible off-target effects. Therefore, it is crucial to ensure precise control of the activity of gene-editing systems. This can be achieved by using high-fidelity Cas enzymes and improved sgRNA design tools, as well as by delivering CRISPR mRNA using LNPs in a cell- and tissue-selective manner to improve genome editing efficiency and precision. Additionally, activable genome editing tools, such as eiCRISPR using LNPs, enable selective activation of gene-editing systems in disease cells, thereby reducing the potential for adverse off-target effects. However, the clinical translation of LNP-based mRNA delivery is challenging due to the accumulation of LNPs in the liver after intravenous administration, making extrahepatic mRNA delivery difficult. To overcome this challenge, researchers have developed ionizable lipids and tailored LNP formulations for tissue-specific mRNA delivery to various organs, including the liver, spleen, lung, bone, skeletal muscle, lymph nodes, and tumors. Further studies are needed to deepen our understanding of the structure–activity relationship of lipids and to develop LNPs capable of delivering mRNA in a cell-selective manner.
Acknowledgments
M.W. acknowledges financial support from the National Key R&D Program of China (2017YFA0208100), National Science Foundation of China (22077125) and Beijing Natural Science Foundation (Z220023).
Conflict of interest
The authors declare no conflict of interest.
Biographical Information
Tianyu Ma completed his undergraduate studies at University of Chinese Academy of Science in 2022. He is now a master student in their Institute of Chemistry (ICCAS) under the supervision of Prof. Ming Wang.
Biographical Information
Xianghan Chen completed his master's studies at Anhui Normal University of China in 2019. He is now a PhD student in the Institute of Chemistry, Chinese Academy of Sciences (ICCAS), under the supervision of Prof. Ming Wang.
Biographical Information
Prof. Ming Wang obtained his PhD from ICCAS in 2009. After postdoctoral research at University of Utah and Tufts University (USA), he started his independent career at ICCAS in 2016. His research interests include CRISPR/Cas9 genome editing delivery for chemical biology study.