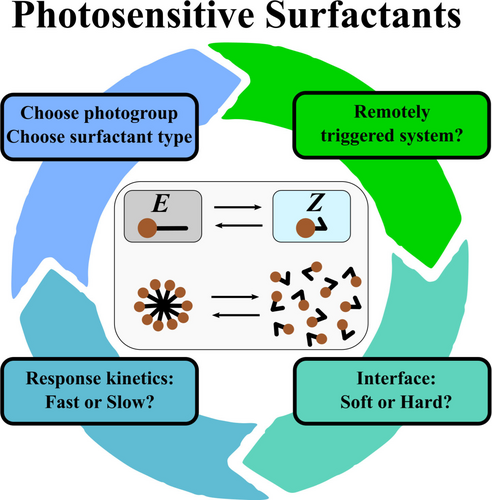
Photoswitchable Surfactants–Are there Alternatives to Azobenzene-Based Systems?
Graphical Abstract
Photosensitive surfactants have gained increasing interest in colloidal science. So far, mostly azaobenzene surfactants have been investigated. In this perspective, we want to address the question, which characteristics ideal photosensitive surfactants should possess. With that, we want to give an outline, how photoswitchable surfactants may be designed, yielding novel, high-performance novel surface-active materials.
Abstract
Owing to their property to alter their surface-activity upon the irradiation with light, photoswitchable surfactants have gained tremendous interest in colloidal science. Their mere addition to a colloidal system allows, e. g., to obtain control over polyelectrolytes, micro- and nanoscale particles or emulsions. Most literature examples focus on azobenzene-based, or related, systems, which employ a photoisomerization reaction for switching. Other structures, such as spiropyrans, play a subordinate role, although they have gained increasing attention over the past few years. In this perspective article, we want to provide an overview about existing systems of photoswitchable surfactants. We address the issue that alternative photoswitches are given less attention, and what benefits surfactants could possess that are based on said switchable units. With our contribution, we want to broaden the view on stimuli-responsive surfactants – and to provide a guideline for the design of novel structures.
Introduction
Surfactants featuring a photoswitchable surface-activity have emerged as powerful materials for the manipulation of macromolecules and colloids in an aqueous environment.1 Consisting of a hydrophobic tail, a switchable unit, and a hydrophilic, electrostatically charged or non-charged, headgroup,1 the molecules’ geometry can be toggled in a light-responsive fashion. This entails an alteration of the surfactants’ molecular surface activity, from a strongly to a less surface-active form, leading to some consequences, which can be readily exploited to manipulate colloidal systems, macromolecules, surfaces, or solutions.
As an example, their switchable surface-activity translates into a conversion of the aggregates these surfactants form in solution, when they are present at a concentration above the critical micelle concentration (CMC). Structural investigations, thus, revealed that the surfactants form different micellar2-8 architectures, depending on their switching state. As the presence of differently shaped aggregates, such as worm-like and spherical micelles, may affect the viscosity of the solution, photo-responsive surfactants represent a suitable platform to adjust the rheological properties of an aqueous system in a light-responsive manner.3, 4 If light-responsive surfactants are mixed with lipids, photo-destructible vesicles may result, whose integrity can be affected using a photo-stimulus.9 In addition, switchable surfactants assembling at liquid/liquid and liquid/gas interfaces, yield switchable emulsions10-12 or foams.2, 13, 14
Along with their switchable surface-activity, photo-responsive surfactants assemble at different interfaces.15, 16 The assembly of the surfactants, which can be studied by different surface-sensitive spectroscopic methods,17-19 has a direct impact of the wettability of the respective surface,19, 20 and can e. g. be illustrated by the behaviour of water droplets on these surfaces.20, 21 The concomitant switching of surface energies can be exploited to control the fouling of bacteria on a surface as well, yielding e. g. tailor-made interfaces with switchable antifouling properties.22
The surfactants’ property to adsorb at interfaces can also be exploited to manipulate micro- and nanoscale objects in water. Objects at the microscale, which are large enough to sediment, can be displaced using the light-driven diffusioosmosis (LDDO) effect.1, 16, 23-27 Briefly, this effect relies on the fact that light irradiation induces a local switching of the surfactant at the particle-water interface. The osmotic pressure, originating from the resulting concentration imbalance near the particle surface, is compensated by a diffusioosmotic flow generated along the surface, which in turn facilitates the particle transport.1, 23, 26, 27
Smaller, nanoscale particles are kinetically stabilized, and can be directly affected in their colloidal stability by photoswitchable surfactants. It has been shown, e. g. for carbon nanomaterials,28, 29 that a surfactant can induce an on-demand flocculation an redispersion of a nanomaterial, depending on the switching state of the molecule. The principle of light-stimulated particle aggregation can also be employed to foster the assembly and disassembly of nanoparticulate superstructures30-33 yielding particle supracrystals that possess, e. g. a photo-switchable magnetic,32 or catalytic33 activity. A decoration of charged particles with surfactants affects the hydrophobicity of the particles. Photoswitching of responsive surfactants can, therefore, mediate the transfer of particles from an aqueous medium to an organic solvent vice versa.34, 35 In recent studies, it has also been shown that photoswitchable surfactants have a direct impact on the morphology of nanoparticles: Particles originating from phase-separated block-copolymers are influenced using light-responsive surfactants, whereby the varying affinity of the surfactant to the different blocks alters the overall structure of the nanostructures.36-39
Surfactants that possess charged headgroups interact electrostatically with their ionic counterpart. This becomes particularly remarkable for polyelectrolytic macromolecules,1, 40-42 referred to as soft materials. The literature on this topic is vast, as reviewed elsewhere,1 and involves studies, where interactions with synthetic polymers23, 43, 44 or macromolecules of biological origin,45-48 such as nucleic acids,46-48 are described. In this perspective article, we want to deduce the concepts, which will foster the design of novel light-responsive surfactants, fueled by different application scenarios. Eventually, we aim to provide a conclusion about surfactant design, which we complement with a careful outlook.
Molecular Architectures of Photoswitchable Surfactants
The Diversity of Azobenzene-Based Structures
Azobenzene is a well-known photo-switch, which has been described in many literature examples. Though being explored as a photo-responsive unit already in the 1930s,49 azobenzenes are still a field of active research, and continuous research efforts have yielded a library of azobenzene structures that have been tuned to cover a broad range of the visible electromagnetic spectrum.50 The peculiarity of azobenzenes is their reversible isomerization ability from an E to a Z configuration, where both isomers differ notably in their electric dipole moment ( ,1, 51 Figure 1a).

Azobenzene, arylazopyrazole and spiropyran moieties and their switching. a) The azobenzene and b) arylazopyrazole (AAP) photoswitch in comparison. c) Spiropyran switching.
Azobenzenes are characterized by a good photostability along with advantageous switching kinetics.52 Beyond that, their straightforward synthetic accessibility renders azobenzene photoswitches the basis for the most abundantly reported light-responsive surfactant systems.1, 53
Classically, the azobenzene-based surfactants consist of a hydrophilic headgroup and a hydrophobic backbone, into which the azobenzene switch is incorporated (Figure 2a). The switching of the system described in Figure 2a occurs, in an aqueous environment, at <400 nm and >450 nm effectively for its back reaction (note, that according to overlapping absorbance peaks between E- and Z-form there is always a relative proportion of both isomers).54 The headgroup may be cationically, anionically or zwitterionically charged, or may consist of a non-charged polar moiety, such as a carbohydrate.1 Alongside, more intricate molecular architectures are known, for instance bola-form or gemini surfactants, which will not be a subject of this article. In this regard, the diversity of azobenzene-based surfactants that consist of two hydrophobic tails should be emphasized, which are the basis for switchable membrane structures.55 Such systems often form complex assemblies, which arouse great interest in their utilization as a material platform. As an example, systems containing a branched tail and a zwitterionic headgroup have been described that transform from worm-like micelles to lamellar bilayers.56, 57
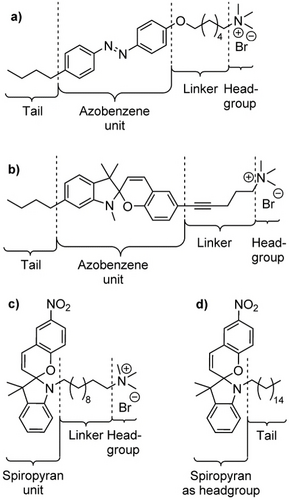
Schematic representation of different photosurfactants. a) An exemplary structure of an azobenzene photosurfactant.44 b) A spiropyran-derived photosurfactant with a molecular architecture inspired by the geometry optimized for azobenzene photosurfactants.11 c) A bola-type spiropyran photosurfactant. Photoisomerization converts the non-charged ω-terminus of the surfactant into a zwitterionic merocyanine.67 d) A spiropyran derivative, which converts into a zwitterionic surfactant during the photoswitching reaction of the spiropyran into a merocyanine headgroup.66
Azopyrazole Systems
Azopyraozles represent photowitchable units that resemble azobenzenes. Their Z to E photoisomerization occurs at 520 nm or 365 nm,19, 58, 59 respectively, whereby this system is characterized by high photostationionary states (PSS) in both directions along with extended thermal half-lives (Figure 1b).19 An analogy to azobenzene surfactants, azopyrazoles have been incorporated into the hydrophobic backbone of a classical surfactant to form photoswitchable surface-active molecules (Figure 1b).2, 18, 19, 60
Spiropyrans
Spiropyrans are outstanding photoswitches, whose transition occurs between a non-charged spiropyran (SP) to a zwitterionic merocyanine (MC) form (Figure 1c). These both forms differ drastically in their dipole moment as reflected in the remarkable difference in their dipole moments of approx. 10 D.61 Its switching mechanism can be considered a two-step process, involving a ring opening and an Z→E isomerization with a C−O bond cleavage (Figure 1c).61 This spontaneous thermodynamic reaction can be reversed by visible light, triggering the E→Z isomerization back to SP. In its open MC form, the phenolic group exhibits weakly basic characteristics.61 Therefore, it can be protonated at low pH values to yield the protonated MCH+ form (Figure 1c). In this form, the zwitterionic MC is turned into a positively charged MCH+ species. The complex switching mechanisms of SP/MC are reviewed elsewhere.61
Possessing three different switching states, spiropyrans are dual photo- and pH-responsive. Beyond that, they also react to many other stimuli. [61] As examples, the switch shows a solvent-dependent behavior (solvochromism),62 is sensitive to the electrochemical potential (electrochromism),63 a mechanical stimulus (mechanochromism),64 or the presence of metal ions.65
Evidently, spiropyrans have also been incorporated in surfactant structures. Evolving from non-charged to charged species, structures are reported, in which the spiropyran is directly coupled to an extended alkyl chain via the nucleophilic nitrogen. These compounds are hydrophobic and develop their surface-active properties when the SP switches to the betaine MC form to function as the hydrophilic head (Figure 2d).66
There are few other structures of surfactants described in literature, which combine a spiropyran moiety with a charged headgroup.34, 66-69 Compared to the aforementioned azobenzene or azopyrazole surfactants, where the spiropyran moiety is a part of the hydrophobic backbone, this class of spiropyran surfactants possesses its photo-responsive unit at the at the -terminus at the surfactant, where it is either an ordinary (in its SP form) or a bola-form surfactant (when switched to MC, see Figure 2c).67 Even though these surfactants have been demonstrated to possess a light-responsive surface-activity, as they have been utilized for the targeted manipulation of silica materials,34, 66-68 we consider this geometry not ideal, as the overall geometry of the molecules does not change notably during the switching. We explain the number of literature examples, however, with the ease of preparation of these structures, since the alkylation of the indole nitrogen in the spiropyran is synthetically straightforward to access. To adapt the geometry that has been optimized for azobenzene surfactants to spiropyran systems, in a previous study, we prepared a surfactant possessing the spiropyran moiety in its backbone (Figure 2b).11 This surfactant showed a good photo-switching behaviour with pronounced differences in their surface activity (as characterized by the difference in critical micellar concentrations of both forms); however, only under acidic conditions. Under neutral and basic conditions, in contrast, the switching was insignificant. This pH-dependency, therefore, renders the surfactant dual-responsive.11
What is the Hallmark of an Efficient Photo-Switch?
The addition of light-responsive surfactants to colloidal systems offers an elegant possibility to render, e. g. suspensions of nano- or microscale particles, emulsions, microgels or polymers, photo-sensitive. In order to design a suitable surfactant, several key properties of the surfactant molecule have to be optimized. Briefly, the interaction of the surfactant with the desired material should differ significantly, depending on the switching state of the surfactant (Figure 3).1, 70, 71
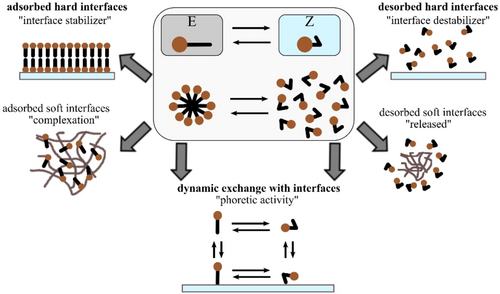
Schematic representation of the processes occurring during the interaction between a photoswitchable surfactant, such as an azobenzene, and soft and hard materials.
At a first glance, this concerns the kinetics of the ad- and desorption of the surfactants as well as the kinetics of their self-assembly into micelles. As the interaction between the surfactant molecules with the desired material or with themselves (by assembling into micelles) is a dynamic equilibrium, one isomer must adsorb quickly and effectively to the material, while the other one is supposed to desorb effectively. The term ‘effectively’ hereby underlines the dynamic nature of the exchange. This renders such systems not always easy to predict, as the kinetics of two isomers interacting dynamically with the material or to the corresponding micelle have to be considered, thus resulting in total 23 =8 possible cross-correlating parameters (Figure 3).
These considerations do not yet comprise the influence of photoisomerization kinetics in the relative isomer ratio that is present in the PSS state. The ratio of both isomers controls the macroscopic structure response of the chemical system and, thus, its light response.49 In the following, we want to deduce the concepts, that would result in the most optimal design of light-responsive surfactants, based on the applications, which is illustrated with general roadmap displayed in Figure 4.

Roadmap to improve photochromism via photo switchable surfactants.
Regarding the surfactant interactions with materials, there are basically two kinds of material interactions to discuss. The first one is based on the physical principle of assembly and aggregation changes within “soft materials,” possessing a diffuse interface, such as polymer chains, networks or brushes. The second one is the interaction of the surfactants with hard interfaces and their structural changes at a molecular scale as well as the macroscopic implications during the photoisomerization.
All the interactions are generally very complex, but they can be roughly approximated by four major key points:
-
During the interaction between surfactant and material, one isomer should strongly remain adsorbed at the interface or within the material matrix, while the second one effectively dissolves in water and, therefore, leaves the material (surface), as depicted in Figure 3. This provides a static change in material response, for instance stabilization/destabilization of particles.28-33
-
Ideally, the surfactant should exchange between the interface/material matrix and the bulk solution in a dynamic, rather quick fashion as reflected in the fact that there is one isomer that adsorbs well to the surface and another one that is well-soluble in water. The isomer that strongly adsorbs to the interface possesses a fast and effective adsorption rate, while the other one desorbs rather rapidly.15, 58 As a result, this kinetic behaviour induces large concentration gradients of the molecules in their different switching states between material/surface and bulk. This dynamic interaction may lead to light-induced phoretic/osmotic activity of micronized objects.16, 23, 24 In addition, in order to prevent a static adsorption of the one isomer at the interface/material matrix, which would render any kind of dynamic interaction literally impossible, the rate of desorption of the well water-soluble isomer should always be larger than the rate of the adsorption of the competitor.
-
The photoisomerization should be ideally occurring at the same time scale or maximum two orders of magnitude slower (or faster, respectively) as the adsorption and desorption rate of both isomers. A too strong deviation between the rates results in a “quasi-static” interaction, meaning, that if the photo-isomerization is very fast, there is only a limited control by adsorption parameters, since the dynamic exchange properties of both isomers is limited by the surfactant-particle interaction rather than the light-stimuli photochemical properties (wavelength and light). This is for example very crucial for photo-induced phoretic activity, where the strength of phoretic activity for soft microparticles23, 24 or the size of the microgel72 cannot be controlled by the applied light intensity at a critical threshold intensity. This is fast photo isomerization rate (~high intensity), where the system enters the adsorption limited regime of photosensitive surfactants for dynamic exchange under illumination.
A slow photoisomerization allows a precise control in light-induced manipulation, but usually, such systems do not allow a strongly pronounced manipulation in time frames of seconds or minutes, which renders them unattractive for most experimental applications. Thus, ideally both photoisomerization rate as well adsorption kinetics should be in time scales of seconds or minutes.
-
For photo-switchable interactions, it is always important to consider the lifetime of the thermodynamically instable Z-isomer, which can range from short to very long time scales (up to several orders of magnitude),73 depending on many factors, such as solvent conditions, functional moiety, and substitutes (~electron donor-acceptor properties).74 In our opinion, ideally, the Z isomer should be rather long-lived, or at least at time scales much longer than the desired photo-switching time (~several hours or longer, while photo-switching usually occurs at time scales of seconds to minutes).54 These conditions facilitate, that the switching between E−Z vice versa can be precisely controlled and that it does not require a permanent illumination of the sample. However, this also depends on the photo-switch. If only one-directional photoisomerization is possible, shorter lifetimes might be beneficial, which still allow a remote triggering between both isomers.
If the lifetime (~1/(thermal relaxation rate)) is in the same time range as the photo-isomerization process, we suggest minimizing the impact of the lifetime by increasing the illumination intensity. Some photochemical reactions may require a critical intensity, as the thermal relaxation is very fast, and effective switching is never reached. A simple approximation of the applied intensity for adjusting the desired isomer molar ratio X in PSS, with respect to the thermal relaxation, can be calculated accordingly:54
,(1)
with X=cE/cZ as the isomer ratio from concentration of E and Z isomer cE and cZ in PSS, kEZ and kZE as the rate constant of photo induced isomerization from E→Z (index EZ) and vice versa (index ZE), kT as thermal relaxation rate constant and I as the intensity of applied light (with λ=constant). Equation 1 shows that with increasing intensity the impact of kT decreases. Thus, in case of low lifetime (~fast thermal relaxation) we suggest increasing the light intensity as much as possible, however, at the expense of an enhanced photodegradation tendency with increasing intensity.
As a general rule of thumb, the surfactant's attractive interaction potential – i. e. its tendency to adsorb – is a result of the hydrophobic effect,75 the surface-activity (correlating with the CMC),76 the packing parameter as well as the polarity of the entire surfactant molecule, comprising the effective dipole moment or the head group type (non-ionic, zwitterionic, ionic).77-80 Note, that all parameters are in strong cross-correlation with each other.
To predict the influence of such complex systems on colloidal systems, thus, in a first approximation, the three parameters should notably differ between the both isomers. However, not always are all changes achievable over the chemical structure. Hence, depending on the material interaction, a trade-off between the polarity or geometry should be aimed at while choosing the surfactant structure and the photo-switchable moiety. This is discussed in more detail in the following for the two major material types: “Soft materials” and “hard interfaces”.
Soft Materials
Soft materials are macromolecular structures formed by polymer chains, polymer networks, i. e. crosslinked polymer chains, and polymer brushes, which are grafted polymers on interfaces. In these types of materials, the surfactant can diffuse into the material (Figure 3). In general, surfactants have a great tendency to adsorb or to form complexes with polymers.81-83 This involves charged polymers, which interact with oppositely charged macromolecules,43-48 such as nucleic acids as very prominent literature examples.46-48
In particular, microgels have gained increasing interest with respect to their interaction with switchable surfactants. The most prominent literature examples of photo-responsive polymer networks are charged microgels, mostly derivates from N-isopropylacrylamide (NIPAAm), co-polymerized with small quantities of charged functional groups,84 which are derived from, e. g. acrylic acid.72, 85 The photo-responsiveness is related to the reversible accommodation and release of the surfactant. It has been pointed out that the CMC of the surfactant within the gel is several orders of magnitude lower than in solution.86 Here, a strong adsorption tendency is observed, when ionic groups along the polymer chain interact with the oppositely charged headgroups of the surfactants. Thus, such dissimilarly charged materials mostly possess the strongest photo-responsiveness. In addition to that, it is has been demonstrated that the interaction is not mainly electrostatically: Also, hydrophobic interactions play a significant role.82 Particularly, azobenzene-containing surfactants turned out to be very effective for controlling remotely the microgel size by light via osmotic pressure variations within the microgel.85 Note, that a similar behaviour is reported also for polymer brushes.23, 87
But what is the reason of the controlled assembly of E- and the disassembly of Z-isomers from the polymer matrix? Compared to the aqueous bulk solution, the “polymer matrix interior” of the microgel is more hydrophobic. This causes a larger energy gain, when the microgel as the “hydrophobic surrounding” hosts the more hydrophobic isomer of the surfactant (i. e. the E-isomer in case of azobenzene surfactants).88 When the surfactant is converted into the Z-form by applying irradiation with UV light, it becomes more hydrophilic and, thus, prefers to stay in the bulk. This results in a release of the Z-isomer from the polymer matrix. For charged microgels, this involves an additional H+ association from bulk solution in polymer matrix.89 Consequently, the osmotic pressure in the polymer matrix changes, which results in a swelling or shrinkage of microgels depending on the absolute bulk concentration.90 As these processes occur osmotically driven, the molecular design of photo-switchable surfactants for such desired photochromism ability of soft materials, one should aim at a strong polarity change during photoisomerization.91-93 In detail, during the photo-switching process of the surfactant, rather the polarity instead of geometry should alter, which is already well satisfied for well-known azo-benzene, azo-pyrazole, spiropyran, surfactants.92, 93
Hard Interfaces
The dynamic transition between the isomers of a photo-switchable surfactant at the surface represents the fundamental physical principle for the manipulation of materials possessing a hard interface. As an example, plain surfaces can be affected with respect to their wetting behaviour,19-21, 94 colloids can be aggregated and dispersed on demand,28-33 or microscale particles are transported on a solid surface in the presence of a surfactant.1, 26, 27 These manipulations have their fundamental physical principle on the complex interaction of surfactants with interfaces on molecular scale. For such interfacial-bulk solution interactions, there are two general key parameters which need to be tuned.
First, (i), the general molecular structure of the surfactant determines the exchange ability of the molecule between the interface and the bulk solution. Here, the size of the surfactant plays an important role. Smaller molecules can more easily align as mono- or multilayers, hemi- and full micelles at smooth or rough interfaces or at porous structures than larger ones, as they are sterically less demanding.15, 95-97 Their tendency to be attracted by the interface is also driven by the properties of the head group98 but also by the chain length of hydrophobic tail,99 which is in turn characterized by the packing parameter of the entire molecule.15, 100 The adsorption, however, is not only determined by the steric properties of the molecule itself. As dynamic processes allow generally a faster exchange between the interface and bulk solution, the properties affecting the ad- and desorption kinetics must be considered as well. This may include the surface potential of the interface or the counterion type of the interacting ion pairs, i. e. counterionic specific interactions.101
Second, (ii), the packing parameters of both isomers should be strongly different. Therefore, the photo-switchable moiety should impart a strong structural change of the entire geometry of the surfactant during the switching. As rule of thumb, an indication of the surface-active potential of surfactants is the concentration, at which the surfactant assembles from entirely water-soluble molecules into micelles, i. e., the CMC.100 This number provides information, at which concentration the surfactant molecules strongly adsorbs at interfaces. Values of CMC can vary in several orders of magnitude depending on the length and structure of the hydrophobic chain length and head group.102 Generally higher chain lengths of the backbone and less polarity of the headgroup results in lower values of the CMC,103 as these parameters increase the hydrophobicity of the entire molecule and the tendency to aggregate in comparison to be surrounded from water molecules.
Both isomers of the photo-switchable surfactant are surface-active, translating into different absolute bulk surfactant concentration. For a reversibly switching “on-off” surface activity, one isomer should always have a much lower CMC value than the corresponding isomer and ideally, they differ as much as possible between both isomers.1 Such properties are satisfied for surfactants exhibiting an azobenzene, or azopyrazole (AAP) moiety, where the difference is approximately around one order of magnitude between E- and Z-isomer.60, 102 Due to the enhanced water-solubility of the Z-isomer, its CMC is higher. Empirically, the difference in CMC between E- and Z- surfactants for azobenze may be approximated as a formal difference in the alkyl chain length by three methylene groups (12.6 carbon atoms for E- to 9.7 carbon atoms for the Z-isomer) as example from a reported substance.104 Discussed findings represent an overall guideline but are not universal and also strongly depend on the nature of the head group and also the position of the photo-switchable unit along the hydrophobic chain.105
When it is aimed to manipulate hard surfaces, the surfactant should be added at such a concentration, the CMC of the Z-isomer (CMCZ) is not reached yet, but that it is already above the CMC of the E-isomer CMCE with CMCE<c<CMCZ (c being the working concentration), with the tendency to adjust the total surfactant concentration closer to the CMC value of the surface-active E form. If the concentration is much lower than the CMC range, the response is limited due to a slow and dynamic exchange. The same is also important for concentrations exceeding the CMC of the well water-soluble Z form, since in this case, both isomers are rather surface active at this “high” concentration range, and the light-induced surface-activity contrast between both forms is rather unpronounced.
Despite the fact, that low-CMC surfactants are quite surface-active, the utilization of surfactants with overall high CMC values are more favoured. The rate of adsorption is a product of the adsorption rate constant and the isomer concentration, k⋅c,15, 72 which is a simple parameter to control, to amplify the dynamics of interaction towards higher concentration. Accordingly, ionic surfactants are always a better choice, as they possess generally higher solubility, thus, increased values of CMC (~0.1–1 mM) compared to non-ionic derivates (~10−3 to 10−2 mM)106-109 at fixed hydrophobic chain lengths. However, this depends on counterion-specific interactions between head group and ions101 as well as strongly on the ionic strength of the bulk solution and applies merely for low ionic strength solution (Millipore water instead of a buffered solution).
Note, that the CMC for ionic surfactants strongly decreases with increasing concentrations of salt in solution. If the medium must be buffered, the only choice is restricted to non-ionic surfactants.
What molecular features does the surfactant need to exhibit to be well suited for the manipulation of hard interfaces? In order to maximize the CMC difference between two isomers, the weighting on molecular tuning should rely on alterations of via the packing parameter.1 It can be pointed out here, that the molecular geometry can play a more important role than molecule's polarity, which can be hypothesized from comparing azobenzene vs. spiropyran surfactants (Figure 5). In detail, the azo-benzene isomerization from E to Z results in a drastic change of the angular reorientation from an effective zero angle (0°, E-form) into an acute angle (20°, Z-form), which basically changes the geometry of the hydrophobic tail from an elongated shape into a bent shape as illustrated in Figure 5b.15 For spiropyrans, however, the transition between a non-charged spiropyran to a zwitterionic merocyanine results in a rotation along the molecular axis of the surfactant (Figure 5d) and then the effective geometry is similar between both forms. Even though these both forms of the photo moiety siginificantly differ in their dipole moment (~3D for azobenzenes vs. ~10 D for spiropyrans),61 they possess similar ΔCMC values. If the polarity would dominate the tendency of self-assembly, drastic changes in the CMC difference for the spiropyran in comparison to azobenzene containing surfactants could be expected. Based on these observations, we hypothesize, that the geometry change has a much stronger impact in comparison to polarity, which causes the huge difference of CMC between E versus Z form for azobenzene and obviously not for spiropyran versus merocyanine. Further, the position and the chain length of the hydrophobic spacer and tail influences the value of the CMC, where the tail has a stronger impact on CMC.102
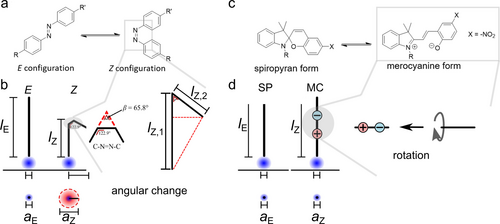
Comparison between effective geometry change from (a,b) azobenzene containing surfactant; (c,d) spiropyrene based surfactant. (a,c) chemical structure of photo moiety. (b,d) Schematic representation of an effective geometry of the surfactant adsorbed with the headgroup perpendicular towards an interface. Bottom circle illustrates effective surface coverage. The photo-moiety assumed is incorporated in the centre of the hydrophobic chain. The headgroup is approximated as trimethylammonium bromide.10, 65
Are there Other Possible Photoswitch Motifs?
Various strategies have the potential to improve the performance of photoswitchable surfactants. Taking the lessons learnt for spiropyrans and azobenzens, a selection of promising motifs and candidates will be highlighted and briefly discussed. This will include both new classes of photoswitches as well as modifications of established ones.
As previously discussed, both the polarity and the geometry differences between the two isomers are important parameters to consider, if an optimal manipulation of colloids is targeted. As shown in Figure 4, the significant alteration of these properties among the photoisomerization is the crucial lever to improve the ▵CMC. Only the combination with the photochemical properties of photoisomerization allows a comprehensive description of the photoswitchable surfactant. An ideal photoswitch converts a large quantity of absorbed photons into a chemical reaction (high quantum yield), resulting in an almost full conversion of one isomer at the PSS. This means that at given wavelength of illumination, most isomers in the E state have to be transferred into Z and vice versa at another wavelength. Only the combination of both properties (pronounced ▵CMC and almost full isomer conversion) enables a precise control of alteration between E- and Z-surfactant and, thus, into an alteration of the switchable response of colloidal systems (nano- and microparticles, soft particles, polymeric networks etc.), which in our opinion represents the most ideal photo-responsive surfactants.
Moreover, the thermal stability, absorption wavelength and molar absorption coefficient are important parameters to consider, which depend on the application scenario. For example, the molar adsorption coefficient determines the applicable exposure wavelengths and may limit the usage in biological processes (~UV light), while technical processes with a demand for high spatial resolution are benefiting from rather shorter wavelengths.
Photophysically, the absorption features, characterized by the absorption coefficient μa, need to be optimized to obtain an ideal response of the molecule to the incident light. This parameter constitutes an inherent molecular property and is used to be determined by default in studies, concerning molecular photoswitches. In addition to the actual absorption characteristics, in a colloidal system, also the scattering properties must be considered. Along with the targeted manipulation, such as the swelling and shrinking of a microgel, the aggregation of nanomaterials, or the formation of micelles from fully dissolved species inter alia, the switching process of a surfactant changes the system from a non-scattering into a scattering sample vice versa. Hence, also the scattering coefficient μs of the respective colloidal system has to receive attention. It must be mentioned, that the absorbance of the non-scattering state requires precise engineering, so that the spectral absorption and the intensity loss due to scattering do not interfere with each other. This will avoid total absorbance and ensure a homogeneous photoreaction throughout the material. The structure-property relationship of both absorption wavelength and molar absorption coefficient of photoswitches as well as the chemical engineering of surfactants are discussed comprehensively elsewhere.110, 111
As discussed in the previous section, both increasing the polarity and geometry change upon the switching process determines the performance of the photoswitchable surfactant. Azobenzenes have long been established as photoswitches and can therefore be considered a benchmark system. To improve their performance, the change of the molecular polarity upon E to Z transition can be tuned. Improving this polarity change, however, represents a challenging task. Altering the substitution pattern can address this issue, yet will almost always alter the electronic structure of the azobenzene, and with it the photochemistry. Particularly, the thermal stability of the Z state is well known to rapidly decline throughout substitution with that push-pull patterns.112, 113 Focus of the past decade was shifting the absorption into the visible, and even the IR region.50 Further, the applicability in water was a focus.114 With this momentum, various substitution patterns have been investigated. Garmshausen et. al reported the replacement of one benzene moiety in the azobenzene by an tetracyanocyclopentadienide (Figure 6a).115
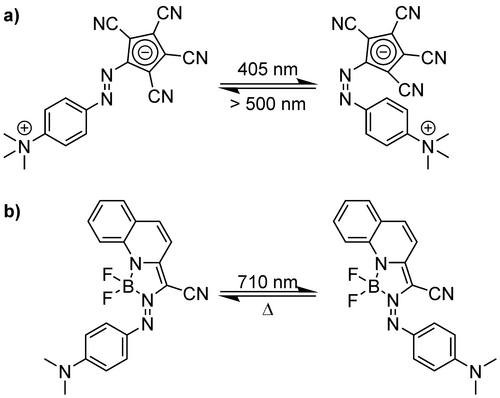
Improving double-bond isomerization at an N=N bond throughout enhanced azobenzene motifs: a) zwitterionic azobenzene incorporating a quaternary ammonium cation and a stabilised cyclopentadienyl anion; b) azobenzene-difluoroboron complex (azo-BF2).
When the second benzene moity is substituted with an ammonium group, a photoswitchable zwitterion is formed. It shows a comparable photochemical behaviour to frequently used azobenzenes, and the absorption maxima (λmax E=390 nm and λmax Z=470 nm in acetonitrile) are well separated. Illumination of the E-isomer with 405 nm results in a PSS of 91 % of the Z-Form, and a reversion upon illumination with light greater than 500 nm of 94 % E-isomer. The half-life time is reported to be 150 min.115 Alkyl substitution at the nitrogen could access novel surfactant types. These would be expected to alter the geometry of the head group rather than their polarity, which could be beneficial for the interaction with hard materials.
Another very potent variation of the azobenzene is the azobenzene-difluoroboron complex (azo-BF2) switch (Figure 6b).116 Here, a difluoroboron is coordinated to phenylhydrazones. They can be isomerized between the E isomer, which is thermodynamically stable, and the Z isomer. These switches can show excellent PSSs and very high photoisomerization quantum yields (PSSE−Z=97 %Z, PSSZ−E=80 %E, ΦE−Z=0.48, ΦZ−E=0.67 in dichloromethane – DCM). The half-life of the Z form was approximately 12.5 h in deoxygenated and 0.5 h in regular DCM. These represent convenient photosystems, which can, upon a selective alkylation at the two benzene rings, yield surfactants, which experience a large change in their geometry upon switching. This feature would render the surfactants well suited for an interaction with hard materials.
Alongside azobenzenes, various other motifs show an E−Z isomerisation (Figure 7).114 Even the merocyanine can be modified to exhibit solely an E−Z isomerisation. Other moieties are stiff stilbenes (Figure 7a),117 indigo and thioindigo118, 119 (Figure 7b) as well as hemi-indigoid120, 121 (Figure 7c) and aryliminoindigoid122, 123 (Figure 7d) and hydrazone124-127 (Figure 7e) photoswitches. As these structures promise a pronounced geometry change, surfactants derived therefrom would interact well with hard materials.
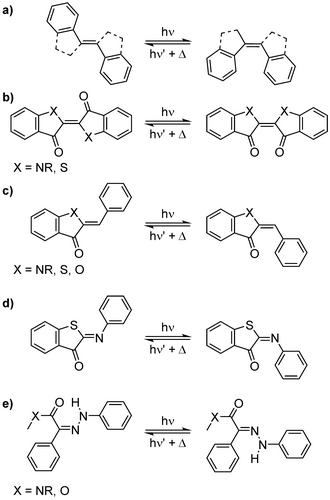
Alternative switching motif to induce a significant geometrical change through double-bond isomerization (C=C, and C=N): a) stiff stilbenes (Figure 7a), b) indigo and thioindigo as well as c) hemi-indigoid, d) aryliminoindigoid and e) (aryl) hydrazone.
Multiresponsive chromism is in our opinion a promising strategy to achieve a significant change in both geometry and/or polarity. Here, a set of equilibria are coupled, which can be of thermal of photochemical nature, and used to maximize the alteration of the molecular properties. In fact, the conversion between spiropyrans and merocyanine is one of the most complex examples for multi-responsive chromism. It does not only comprise an electrocyclization, but also a E−Z- isomerization as well as an acid-base equilibrium.61 This makes it such a potent switch for photoswitchable surfactants.128 However, these features render it also vulnerable to external interference, e. g. solvent polarity or pH. In particular, water is a challenging environment. Alongside spiropyran, a variety of motifs are incorporating both E−Z-isomerisation with electrocyclization, e. g. fulgides, fulgimides,129-135 chromenes, naphthopyrans, dihydroazolenes and stiff diarylethens.136, 137 These interconnections result not only a significant geometric changes, but also in more rigid structures of the photoproduct.
An alternative strategy to maximise geometric change among switching is the coupling of multiple E−Z-isomerisations, e. g. by combination of multiple azo-unites in one molecular moity (Figure 8).138, 139 High conversions to the Z,Z configuration are achievable, but only if the two photosiwchable units are π-decoupled. This can be achieved throughout a dihedral angle of 90° as it is shown in the example by Bléger et al.138 Here, illumination with 368 nm results in 95 % Z,Z configuration. In asymmetric molecules, where the azobenzene moieties are not identical140 or combined with other switches,141 it is possible to address both switches independently. Cross-conjugation, e. g. by substituting the ortho positions is an alternative approach to decouple the azo units. Using this pattern even photoresponsive foldamers are reported.142, 143 The switching states of photosurfactants derived from this moiety would tremendously differ in their geometry and could, thus, be highly interesting possessing a high ΔCMC.
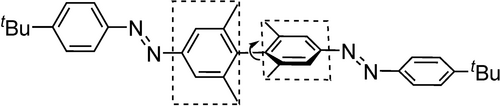
Maximizing geometry changes upon coupling of azobenzenes.
Moreover, the combination of photochemical with a thermal equilibrium, such as a protonation/deprotonation and thermal equilibrium in one molecule can be achieved. Donor–acceptor Stenhouse adducts (DASAs) are a new and promising class of photoswitches, first described in 2014.144, 145 Visible light induces a E−Z-photoisomerization followed by a thermal cyclisation and proton transfer (Figure 9). This cascade of reactions ends in a significant change in geometry and a formation of zwitterion. Thereby, this class combines the preferable properties of azobenzenes and spiropyrans. In addition, they are synthetically straightforward to access. Consequently, first applications to functionalise polymers, e. g. in microgels or in liquid crystals have been reported.146, 147 Incorporating this photoswitch into a surfactant structure would therefore result in surfactants whose isomers differ in both geometry as well as polarity, which renders the respective surfactant an ideal candidate for the manipulation of both hard and soft materials.
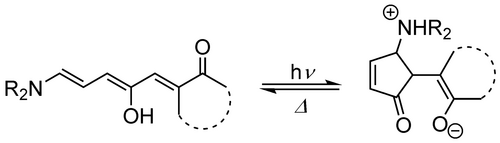
Switching motif of Donor–acceptor Stenhouse adducts (DASAs).
The photochemical electrocyclization of diarylethenes results in a conversion of a hexatriene into a cyclohexadiene. This is accompanied by a relocalisation of double bonds, which has been used to stabilise or destabilise, respectively, the charges in (hetero) aromatic moieties (Figure 10). Gurke et al. incorporated a thiozolon moiety into a diarylethenes allowing for the modulation of the pKa.148 A negative charge is well stabilized in the aromatic thiazolon. Upon photoisomerization, the aromatic stabilization of vanishes, which goes along with a significant increase of the pKa. The ring closure can be addressed with UV light, leading to an almost full conversion into a thermally stable isomer. Upon illumination with visible light reverses the reaction. Azulene moiety can be used in a similar fashion to control a protonation, forming a cation.149 Incorporating this moiety into a surfactant could result in structures whose switching states differ in the polarity of a surfactant by controlling a protonation state. This could be highly interesting for the remote control of soft materials.
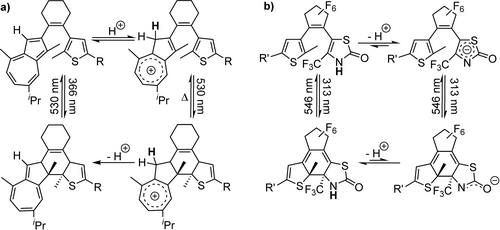
Interconnecting acid-base reaction with photochemical equilibria of diarylethene. An electrocyclic ring-closure results in destabilizing of a charge due to removal of aromaticity of an a) positively charged azulene or b) negatively charged thiazolone moiety; R=p-(trifluoromethyl) phenyl, R’=p-(methoxy) phenyl.
Conclusion and Outlook
Photoresponsive surfactants can be used for the manipulation of colloids, with soft materials, such as linear and cross-linked polymers, and hard interfaces. Photoswitchable surfactants can be designed in such a way that their two corresponding isomers possess either pronounced differences in their polarity, or a distinct change in the surfactant's molecular geometry. Whereas large polarity changes give rise to an optimal interaction with soft materials, changes in the surfactant's molecular geometry translate into notable CMC differences, which is particularly interesting for the manipulation of hard interfaces.
Despite the broad interest in such materials, literature provides chiefly examples which are based on azobenzene-, azopyrazole-, or spiropyran-derived molecules. In this article, we pointed out that alternative photoswitches exist, which could be implemented into the structure of a photoswitchable surfactant. With this article, we provide a careful outline, how these switches can be incorporated into a surfactant molecule, and what properties of the respective photoswitchable surfactants can be anticipated with respect to different applications.
Acknowledgments
The authors thank Prof. Dr. Alexander Böker and Prof. Dr. Svetlana Santer for continuous support. M. R. acknowledges the German Research Foundation (DFG) for funding (project number 471323994). M. B. also thanks the German Research Foundation (DFG) for funding (BE 7745/1-1, project number 469240574). Open Access funding enabled and organized by Projekt DEAL.
Conflict of Interests
The authors declare no conflict of interest.